
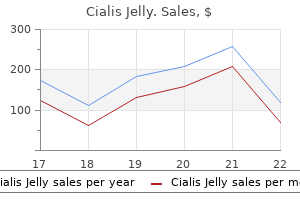
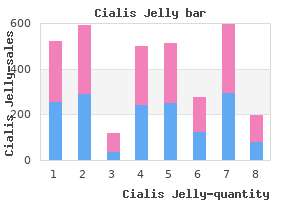
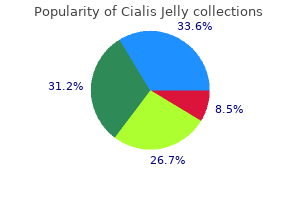
Cialis Jelly
| Contato
Página Inicial

"20 mg cialis jelly discount mastercard, erectile dysfunction korean ginseng".
C. Rasarus, M.A., Ph.D.
Associate Professor, Homer G. Phillips College of Osteopathic Medicine
The sublimation fee again takes the type of a circulate term equaling a driving drive divided by a resistance: Sublimation fee = (Pi - Pc) Rp the place Pi is the vapor stress of ice at the sublimation entrance erectile dysfunction medications in india order cialis jelly 20 mg on line, Pc is the partial pressure of water vapor in the chamber erectile dysfunction 2014 trusted cialis jelly 20 mg, and Rp is the resistance of the partially dried layer of solids testosterone associations with erectile dysfunction diabetes and the metabolic syndrome 20 mg cialis jelly purchase amex. Since the sublimation entrance moves from the highest of the vial to the underside throughout main drying erectile dysfunction drugs free sample cialis jelly 20 mg without prescription, the depth of the partially dried layer increases and the resistance increases. This causes the sublimation price to decrease and, since the fee of warmth move from the shelf remains roughly fixed, the product temperature increases. Of course, this also will increase Pi and will increase the driving pressure for sublimation. Thus, underneath the identical set of primary drying conditions, the product temperature profile can range broadly relying on the resistance traits of the formulation. As resistance to mass switch will increase, management of product temperature turns into extra unsure. For formulations that have a comparatively excessive resistance to circulate of water vapor, it is necessary to limit the depth of the fill. It is usually a great follow to restrict the fill volume to no extra than about onethird of the capability of the vial. Fast freezing leads to small ice crystals that, in turn, have a relatively high resistance to mass transfer. Instead of freezing the product at atmospheric strain, as is often accomplished in vial freeze-drying, the freezing process is carried out at an elevated strain of maybe 15�18 psig. When the product reaches a predetermined temperature, the pressure is quickly released. This ends in ice nucleation in all of the vials on the same time, the place nucleation proceeds from the top of the fill volume to the underside. Of course, reasonably uniform distribution of the ice fog is necessary, such that at least one ice crystal is launched into every vial. As of this writing, neither technique has but been extensively adopted for commercial manufacturing operations. Changes in morphology of the partial dried cake during major drying may end up in modifications in resistance of the dried product layer to the flow of water vapor. Microcollapse leads to holes appearing in plates of amorphous substance, with an accompanying lower in resistance of the dried layer. This would be anticipated to result in an elevated sublimation fee and a lower in product temperature during primary drying. The term "microcollapse" also can refer to collapse on a microscopic scale occurring in a formulation containing a mix of amorphous and crystalline components. In this case, the crystalline element may be considered a "scaffold" around which the amorphous material can collapse. Measurement of Sublimation Rate the sublimation rate can be measured in several methods. If a sample thief is available to take away samples from the freeze dryer in the course of the course of, then several vials can be pre-weighed and recognized. The thief is then used to take away these vials at varied occasions during the major drying process, reweighed, and a weight loss vs. If no thief is on the market, the same method can be used besides that the cycle is terminated earlier than major drying is accomplished. Of course, this can be a destructive check, and only one drying time point is feasible, but the vials 924 can be reweighed, and a mean sublimation rate over the time interval may be calculated. Parenteral Medications connecting the chamber with the condenser in freeze dryers with external condensers (33). Water vapor flows via this cylindrical duct because the upstream pressure, Pu, is greater than the downstream stress, Pd. As water vapor flows through this duct, the stress decreases and, since the mass flow fee is constant for any axial place alongside the duct, the rate of the vapor will increase. As the speed of sound is approached, additional discount of the pressure on the condenser aspect of the duct will cause no change in the mass circulate rate via the duct. This represents the maximum sublimation price that the freeze dryer will help at any given chamber strain. Attempting to operate at a higher sublimation fee would end result within the inability to management chamber strain. Patel and coworkers (34) later demonstrated that the pressure ratio between the chamber and the condenser could be a useful measurement to indicate the onset of choked move. The quantity of this unfrozen water relies upon largely on the nature of the formulation. In formulations with a comparatively excessive content of amorphous solid, the unfrozen water stage is relatively excessive. Since ice is not current throughout secondary drying, higher shelf temperatures are usually used as compared with major drying. The fee of water elimination throughout secondary drying is decided by, not surprisingly, the formulation composition, in addition to the shelf temperature. For the formulations examined, secondary drying seems to take place in two stages-an early "quick" part, followed by a "gradual" phase, the place a plateau is reached in the residual moisture as a function of drying time. This plateau stage is decided largely by the shelf temperature throughout secondary drying. Rate of secondary drying was shown to be, no less than for the model techniques studied, impartial of the chamber stress. This is counter to the frequent viewpoint that the chamber stress should be decreased to the bottom sensible attainable degree throughout secondary drying, and supports the concept that the rate limiting step in secondary drying is both diffusion of water through the glassy matrix or evaporation of water on the surface of the solid-most probably the former. While this is essential when developing freeze-dry cycles, it has significant drawbacks as a monitoring technique in a producing setting. This results in lower degrees of supercooling, larger ice crystals, and quicker drying rates. In basic, monitored vials endure sublimation at a rate roughly 10% quicker than non-monitored vials. Second, placing thermocouple probes in individual vials is a guide process that inevitably compromises sterility assurance. Some producers attempt to keep away from this by putting monitored vials in the front row of vials, closest to the chamber door. However, as discussed above, this place is essentially the most topic to the "edge impact," making information from monitored vials even more nonrepresentative. Finally, advancing know-how in parenteral manufacturing has made automated loading/ unloading systems common in freeze-drying. Such techniques make it much more tough to place temperature measuring units in individual vials. While not widely used at this point, early analysis work by Schneid and Gieseller (35) as nicely as Nail and coworkers (36) reveals promise for utility of this expertise in pharmaceutical freeze-drying. There is a continuing want in the business for better course of monitoring instruments, and the past a number of years have seen appreciable activity in process monitoring tools. Below is a brief survey of methods supposed to monitor the standing of the whole batch. Choked Flow in Freeze-Drying Normally, the resistance of the dried product layer is the controlling resistance to mass transfer in freeze-drying. However, under aggressive drying conditions, Searles demonstrated that one other resistance can become dominant, this one arising from the duct 1 1-F zero. Freeze-Drying 925 for monitoring freeze-drying is predicated on capacitance adjustments due to sorption of water vapor. Comparative Pressure Measurement Comparative stress measurement is based on using two forms of stress sensors-a capacitance manometer and a thermal conductivity-type gauge (a thermocouple gauge or, extra generally, a Pirani gauge) (36). The capacitance manometer relies on capacitance changes related to a versatile metal diaphragm between a sealed reference cell and the method gases. The thermocouple-type gauge, on the other hand, is preferentially sensitive to water vapor because of the higher thermal conductivity of water vapor relative to nitrogen or oxygen. During major drying, the obvious stress as measured by a Pirani gauge is almost fixed, and is considerably greater than the "true" stress as measured by capacitance manometer, because the composition of the vapor in the chamber is almost all water vapor. As main drying ends and the partial pressure of water vapor decreases, the Pirani reading decreases.
Clottable protein in Limulus: its localization and kinetics of its coagulation by endotoxin erectile dysfunction protocol review article cialis jelly 20 mg otc. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation erectile dysfunction causes prescription drugs cialis jelly 20 mg discount overnight delivery, cutoff development drugs for erectile dysfunction philippines buy cialis jelly 20 mg cheap, and efficiency in patients with Acute Myelogenous Leukemia and Myelodysplastic Syndrome erectile dysfunction levitra discount cialis jelly 20 mg with mastercard. Endotoxin contamination of parenteral medicine and radiopharmaceuticals as decided by the Limulus amebocyte lysate methodology. Biomedical Applications of the Horseshoe Crab (Limulidae), Progress in Clinical and Biological Research, vol 29. An invertebrate coagulation system activated by endotoxin: evidence for enzymatic mediation. Validation Status of Five In Vitro Test Methods Proposed for Assessing Potential Pyrogenicity of Pharmaceuticals and Other Products. Genetic engineering strategy to develop next-generation reagents for endotoxin quantification. Bacterial Endotoxins-Test Methodologies, Routine Monitoring and Alternatives to Batch Testing. European Pharmacopoeia policy on bacterial endotoxins in substances for pharmaceutical use, Pharmeuropa, Useful data, September 2014 (revised February 2015), European Pharmacopoeia Commission, Strasbourg, France. Bacterial endotoxin testing: a report on the methods, background, data, and regulatory history of extraction recovery effectivity. Biological evaluation of medical units Part 1: Evaluation and testing within the danger management course of. Biological evaluation of medical gadgets Part 12: Sample preparation and reference materials. Low lipopolysaccharide restoration versus low endotoxin recovery in frequent biological product matrices. An advanced endotoxin assay insights and methods for overcoming low endotoxin restoration in advanced formulations. Reliability of endotoxindetection mechanistical principles of endotoxinmasking and strategies for demasking. Presentation at the Parenteral Drug Association Conference; October 2014: Berlin, Germany. Ultrafiltration to remove endotoxins and different cytokine inducing materials from tissue tradition media and parenteral fluids. Reduction of endotoxin in a protein combination utilizing sturdy anion-exchange membrane absorption. Comparison of bacterial and endotoxin retention by charge-modified sterilizing grade filters throughout intermittent long-term use. Effects of warmth and chemicals on erythrocyte-modifying, antigenic, poisonous and pyrogenic properties. Alteration of physical, chemical and biological properties of endotoxin by treatment with delicate alkali. The price of endotoxin destruction during water therapy using a mixture of ozone and ultraviolet radiation. The check first appeared in 1932 [1] and included the basic features of the modern test-two media, prescribed dilution scheme (for bacteriostasis/fungistasis or technique suitability), and an outlined incubation time. The unique test had the media incubated for five days and allowed two retests (all three checks had to show growth for the sterility check to fail). This take a look at has generated controversy as to its position in product high quality testing for many years. How did we come to think that this check was designed to assess the sterility of the product Recovery of viable cells from the filter(s) is carried out by submerging the filter in one of two restoration media, or including medium to the apparatus itself, followed by incubation at specified temperatures for not lower than 14 days. The second test includes the direct immersion of the product or suspensions into an appropriate quantity of the two media to permit progress. The media are designed to assist growth in an aerobic surroundings or progress in an setting of restricted oxygen availability. This check requires demonstration that the specific methodology used is suitable for that product. As early as 1956, Bryce revealed an article describing the 2 critical limitations of this take a look at. He put ahead that the test was restricted in that it could possibly only acknowledge organisms able to grow underneath the conditions of the check, and that the pattern size is so restricted that it offers only a gross estimate of the state of "sterility" of the product lot [2]. There have been several adjustments within the compendial sterility test since that point, culminating in the internationally harmonized check [4]. The assertion shall indicate the situation of knowledge that set up that the methods used in the testing of the pattern meet proper requirements of accuracy and reliability as applied to the product examined. The suitability of all testing strategies used shall be verified under precise circumstances of use. The compendial sterility check has significant limitations as a product high quality check. The Biologics check is analogous in basic aspects to the compendial sterility checks. Further, handling of a higher number of samples would enhance the danger of a false constructive check as a outcome of extraneous contamination. Changes of this kind seem unlikely within the compendial Sterility Tests at this time limit. A dialogue of different sampling plans that could be used was presented by Bryce [2], and a full dialogue of the controversy over the final resolution of the current process is provided by Bowman [3]. After extensive evaluation, all the proposed sampling plans have been found wanting for one reason or another. The Compendial Sterility Tests One incessantly ignored facet of discussions of sampling plans is that the statistical analyses assume that the test system would recover even a single microorganism if it were current in the sample. These media and their corresponding incubation temperatures were chosen to maximize recovery of potential contaminants early within the development of the tests. However, some authors have questioned the selection of media [6], while others have suggested that using strong media somewhat than liquid media could be applicable [7]. The selections within the current harmonized process mirror those media to which all events within the harmonization course of might agree. Similarly, the Pharm Eur third Edition [11] allowed a 7-day incubation period (unless mandated otherwise by local authorities). This allowance was amended in 1998 with the 4th version to 14 days incubation [12]. More recently, this place was repeated with retrospective information supplied by German and Australian staff who wished to ensure that a harmonized process included an incubation period of a minimal of 14 days [14,15]. Equipment Commonly Used for the Sterility Tests Traditionally, the sterility take a look at has been performed in a clear room maintained on the equal of grade "B" situations using an enclosure similar to a horizontal laminar airflow cabinet that meets grade "A" conditions to minimize the potential for false positives. The consideration here is that incubators used within the microbiology laboratory commonly contain both petri dishes or liquid cultures that support excessive numbers of bacterial cells. The use of this identical incubator for sterility testing is chancy at finest, and if the situation arose, the investigation of a sterility check constructive outcome would turn into hopelessly confounded by the untestable suspicion that the take a look at became contaminated within the incubator. It is far better to reduce any potential for laboratory contamination-in this instance by way of the utilization of dedicated incubators for the sterility take a look at restoration media incubation. The anathema of a false sterility constructive drives many gear and procedural decisions within the sterility tests. Several specialised items of apparatus have been developed for the membrane filtration sterility check with the objective in mind of minimizing the potential for adventitious contamination of the test. This system (and others like it) offers one other barrier level to adventitious contamination of the sterility take a look at pattern. When coupled with use of a test isolator system, the chances of false positives within the check turn into vanishingly small (see the section under on Investigations). Now, a retest is simply allowed if the original take a look at was found to be contaminated as a result of a defective methodology or using defective materials, thus invalidating the unique take a look at. Common practice is to use 10 mL of bulk product/media (for a total of 20 mL) for this testing. This informational chapter supplies background in isolator design and construction, the gear qualification issues for the isolator, validation of the decontamination cycle (this would come with the interior setting, the outside of the product containers entering for testing, and the protection of the product from the decontamination cycle), and the maintenance of asepsis throughout the isolator environment. The reader is also instructed that a sterility take a look at carried out in a properly functioning isolator could be very unlikely to end in a false optimistic outcome. This may be true for environmental circumstances; nevertheless, this is most likely not the case if faulty materials, for example, non-sterile media or rinse fluids are used. Finally, instruction is offered on the coaching and safety aspects of the isolator operation.
Cheap cialis jelly 20 mg free shipping. Erection Problems and Different Types of injections.
Over the previous a quantity of years erectile dysfunction treatment yoga generic cialis jelly 20 mg otc, there has been a renewed curiosity in the use of radiation within the preparation of vaccines impotence vitamins supplements cialis jelly 20 mg generic with visa. The reemergence of sure infectious diseases such as tuberculosis might have stimulated this renewed curiosity in vaccines which would possibly be prepared utilizing irradiation impotence divorce buy cialis jelly 20 mg on line. Depending on the microorganism erectile dysfunction protocol free cialis jelly 20 mg buy low cost, the dose of radiation to inactivate the pathogen could additionally be relatively low. For instance, researchers on the University of California, San Diego, have shown that Listeria monocytogenes, a bacterial pathogen, was inactivated at doses as low as 6 kGy, and the irradiated vaccine nonetheless triggered long-term immunity in the vaccinated animals [36]. However, viral pathogens, which generally have considerably higher D10 values than bacterial pathogens, could require a lot greater doses of radiation. Based on research that have been performed over the previous several years, a big benefit of radiation in the preparation of vaccines could reside within the possible formulation of vaccines in a dry state. Proteins Protein drugs are specific and exert their results at low concentrations, and their virtually limitless quantity permits their use to influence a big number of biological processes. Therapeutic proteins include monoclonal antibodies, growth factors, cytokines, soluble receptors, hormones, and proteins that block the function of a wide selection of infectious agents. The capacity of antigenic constructions to elicit immune response is mostly a sequence-dependent property. At this (primary) degree of structural organization, proteins are quite steady to irradiation. Together with the truth that a considerable diploma of denaturation may be tolerated in vaccines, this permits using radiation within the preparation of vaccines. Increasing complexity of buildings generally brings about their increased susceptibility to mechanical, thermal, and chemical stresses. Consequently, terminal sterilization techniques, including heat, gas, and radiation, have historically not been thought of appropriate for parenteral options of proteins [38]. Irradiation in deoxygenated options, on the other hand, favors the reactions of hydrated electrons with peptide bonds and protonated finish amino groups. The former reaction additionally results in fragmentation and the latter to deamination, and each are unacceptable. Unique three-dimensional conformation of proteins (tertiary structure) is maintained by the interactions between amino acid residues which might be distant from one another within the major construction. These interactions include hydrophobic and electrostatic interactions, salt bridges, covalent bonds, and hydrogen bonds. They are sensitive to the presence of water, pH, ionic power, temperature results, radiation-induced modifications of interacting groups, and dissociation of bonds. For instance, an electron adduct radicals shaped by irradiation may switch the electron to a disulfide bond inflicting its discount and eventual collapse of the tertiary structure maintained by that bond. The weakening of the interactions maintaining tertiary structure at an elevated temperature leads to the lack of the tertiary structure (known as denaturation) of proteins on the one hand and to their elevated susceptibility to irradiation on the other hand. For example, a 3 times bigger reactivity of ribonuclease with the hydrated electron has been observed at 65�C as in comparison with the reactivity at 55�C [39]. It has been demonstrated that radiation-induced degradation of functional properties of proteins (enzyme activity) in resolution could be reduced by decreasing the irradiation temperature and by components. For instance, the characteristic e-folding values of doses required for the reduction of an enzyme exercise to 37% of its initial worth (D 37 values) might be elevated by a factor of four if enzymes were irradiated in frozen options at -200�C, as compared to irradiation at 30�C [40]. Other studies have shown that freezing alone is probably not adequate and the addition of antioxidants together with irradiation in the frozen state was wanted to preserve the integrity of the protein at high doses. Addition of scavengers similar to ascorbic acid or oxidized glutathione along with processing at dry ice temperature offered sufficient protection to allow restoration of >90% exercise. It ought to be possible to irradiate proteins at dry ice temperature on a industrial degree with out main constraints. For instance, tissue merchandise are being routinely irradiated at dry ice temperature. However, lyophilization with a well-designed formulation should allow irradiation sterilization to be utilized for terminal sterilization of the drug product. It is feasible that dependence of temperature change on dose price has clouded a few of the outcomes. All different components being equal, greater dose rates will usually lead to a bigger increase within the temperature of the irradiated product. Because temperature can play an necessary position on the response of proteins, it might be answerable for noticed deleterious effects on irradiated proteins quite than dose rate. Conclusion Since the Nineteen Fifties, radiation has been used to terminally sterilize a complete host of healthcare merchandise and plenty of forms of pharmaceutical products including these used in parenteral medicines. Today, one hundred seventy gamma irradiators and 60 electron beam irradiators are getting used around the world for business sterilization purposes. Only a few X-ray irradiators are presently operational, but that may change with time. High-energy electrons from high-power accelerators, gamma rays from radioisotopic sources, and X-rays from accelerator-initiated sources are all capable of penetrating deeply into most supplies, thus effectively sterilizing all elements of the product. Investigations have shown no proof of nosocomial infections which are traceable to the sterilization process. The part on radiation chemistry focused on liquids, which represent a larger challenge to the radiation sterilization process than drug merchandise which are formulated in a dry state. Dry formulations of parenteral drug merchandise are presently being successfully radiation-sterilized on a industrial foundation. Methods for bettering the tolerance of liquid-based parenteral medicines to radiation, together with the addition of antioxidants or radical scavengers and irradiation in a frozen state, are presented on this chapter. With the event of new biologically derived medicine and mixture drug system merchandise, there shall be challenges for effective sterilization of those products-radiation could turn into a most well-liked modality for terminal sterilization of many of those complex products. Radiation resistance of microorganisms comprising the bioburden of working room packs. Existence of collective-excitation power losses from an electron beam passing by way of organic materials. Chemical observe results in condensed systems and implications for biological damage. Radiation Research, Proceeding of the 6th International Congress Radiation Research, Tokyo, May 13�19, 1979. Transient solvated electron, hydroxyl, and hydroperoxy radicals in pulse-irradiated crystalline ice. The effects of radiation on managed drug delivery/controlled drug launch systems, Radiat Phys Chem. Berlin: Bundesinstitut fuer gesundheitlichen Verbraucherschutz und Veterinaermedizin; 2002. Sterilization of Healthcare Products-Radiation-Part 1: Requirements for Development, Validation, and Routine Control of a Sterilization Process for Medical Devices. Sterilization of Healthcare Products-Radiation-Part 2: Sterilization of health care products-Radiation-Part 2: Establishing the sterilization dose. Sterilization of Healthcare Products-Radiation-Part three: Sterilization of Health Care Products-Radiation-Part three: Guidance on Dosimetric Aspects of Development, Validation and Routine Control. Standard Guide for Selection and Calibration of Dosimetry Systems for Radiation Processing. Development of a course of using electron beam for a terminal sterilization for parenteral formulations of prescription drugs, Radiat Phys Chem. Manual on radiation sterilization of medical and biological materials, Technical Report Series 149. Recommended code of practice for radiosterilization of medical products, radiosterilization of medical merchandise. In: Manual on Radiation Sterilization of Medical and Biological Materials, Technical Report Series No. A new improvement: Irradiation of freezedried vaccine and different select organic merchandise. Reactions of proteins with hydrated electrons: the impact of conformation on the response fee constant. Protein modification brought on by a high dose of gamma irradiation in cryo-sterilized plasma: Protective effects of ascorbate. Preformulation research oriented towards sustained delivery of recombinant somatotropin.
According to 2016 sales data erectile dysfunction medication new 20 mg cialis jelly generic free shipping, of the 5 largest promoting drug products on the planet muse erectile dysfunction wiki cialis jelly 20 mg purchase without prescription, 4 are therapeutic proteins and erectile dysfunction genetic purchase cialis jelly 20 mg free shipping, of those four erectile dysfunction joke cialis jelly 20 mg discount otc, two are available as a freeze-dried strong in a vial (1). This is a testament not only to the dominant position of proteins as therapeutic brokers but additionally to the critical nature of freeze-drying within the manufacture of those medication. Freeze-drying takes place because of sublimation, where water converts from the stable state to the vapor state without first turning into a liquid. This can only happen below the triple point of ice, which is at a temperature of about 0�C and a stress of about four. This strain refers to the partial pressure of water vapor, not the whole system strain, so sublimation of ice can happen at atmospheric pressure as long as the partial stress of water vapor is less than about four. Everyday examples of sublimation of ice embrace ice cubes shrinking over time within the residence freezer, as well as "freezer burn" attributable to local sublimation in frozen food products. In order to be a sensible course of commercially, the system strain should be maintained under the vapor strain of ice, so that the water vapor is transported by bulk vapor move from a area of high strain (the ice surface) to a decrease pressure maintained within the freeze-dry chamber via a condenser maintained at a temperature in the range of -60�C to -80�C. In addition, freeze-drying is an inefficient and costly course of, each in terms of capital price and operating bills. This arises primarily from the excessive warmth enter required to chic the ice (about 2,800 J/g) and the fact that warmth have to be utilized to an evacuated system, making for very poor heat transfer by thermal conduction. Parenteral Medications the purpose of this chapter is to review the scientific and engineering rules important to freeze-drying, and to present an outline of sensible concerns important to both formulation improvement and manufacture of freeze-dried parenteral merchandise. Process Overview Nearly all freeze-dried injectables are aseptically processed (as against being terminally sterilized), where the required quantity of liquid is crammed into previously washed, sterilized, and depyrogenated glass vials. Many modern manufacturing operations avoid placing vials in trays with a backside as a result of this introduces variability in heat transfer. Instead, either removable tray bottoms are used when trays of crammed vials are transported manually or an computerized loading/unloading system is used. The basic parts of the freeze dryer are a heat switch system for eradicating and making use of heat to the product, a condenser to gather the water vapor from the product, and a vacuum system. The shelves of the freeze dryer include inside channels permitting the flow of a warmth switch fluid, usually silicone oil, to control the temperature of the shelf. Freeze dryers for injectable merchandise are sterilized between batches, normally by saturated steam at 15 psig. Most trendy freeze dryers are outfitted with spray nozzles for cleaning-in-place, normally with water for injection. The stack of shelves within the freeze dryer is compressed on the finish of the cycle to force the stopper into its totally seated place. The product is first frozen to a low enough temperature to allow full solidification of the product. The chamber is then evacuated to a strain lower than the vapor pressure of ice (see Table forty two. In order for sublimation to happen at an considerable fee, the chamber stress have to be decrease than this pressure. After the required pressure is reached and the condenser is cooled, warmth is utilized to the shelves to provide the warmth of sublimation of ice. This part known as major drying, the place ice within the frozen material sublimes and flows through the porous mattress of partially dried product into the headspace of the vial, out the open slot in the lyo stopper, and in the end collecting on the condenser. Since much less warmth is required at the slower sublimation price, and the shelf continues to warmth the product, this "further" heat causes the product temperature to enhance considerably. When primary drying is full, the method is usually not over because, in most real formulations, not all the water freezes. In secondary drying, ice is now not present to use the vitality provided by the shelf, and the product temperature increases relatively quickly toward the shelf temperature. As the secondary drying process ends, the product temperature approaches a steady-state worth close to the shelf temperature. Dissolution of the freeze-dried cake should be full, and the reconstitution time should be as fast as attainable. Some high quality attributes could, or could not, be important relying on the meant route of injection. However, this attribute is just crucial for sure routes of administration such as intraspinal, intraocular, or into any part of the brain. Preformulation Considerations the amount of drug per vial for freeze-dried merchandise could range from a couple of micrograms to two or more grams. At low doses, bulking agents are used so that the drug is uniformly dispersed in a pharmaceutically acceptable solid matrix, by which case the freeze-drying traits of the formulation may be determined by the bulking agent. High doses may be more difficult, because the drug itself will in all probability decide the freeze-drying characteristics of the formulation. If the dissolved solids content material within the pre-freeze-dried solution is too low, the appearance of the cake is most likely not acceptable and, more importantly, the dried solids is probably not cohesive enough to forestall ejection of solids from the vial during primary drying. If the focus of dissolved solids is simply too excessive, this may result in issue in process control if the resistance of the dried product layer to the flow of water vapor is simply too excessive, the place a excessive resistance is indicated by a speedy improve in product temperature during main drying. As a really tough guideline, the formulation scientist should aim for a total dissolved solids focus someplace within the vary of 25�150 mg/mL. The quantity of drug, along with its solubility, determines the feasibility of administering the required dose in the applicable volume of answer, and the required quantity is decided by the intended route of injection. For intramuscular administration, up to about 5 mm is injected, and for subcutaneous administration, the injected volume is up to about 1. Of course, many medicine are weak acids and bases, the place the solubility (and usually answer stability) is strongly influenced by pH, so each a solubility vs. Some data is important on each the routes, and the charges, of chemical degradation in resolution. If the drug degrades too rapidly in resolution, then degradation throughout compounding, sterile filtration, filling, and switch to a freeze dryer can current a significant problem. For protein therapeutic agents, physical stability must be examined in answer as well, where bodily stability usually refers to the tendency of proteins to kind aggregates, both soluble or When the product is sufficiently dry, the stoppers, which were partially inserted after filling the vials, are inserted into the fully seated place by means of a hydraulic system which compresses the stack of shelves. It is common apply to stopper the vials whereas the chamber is still under a minimum of partial vacuum, because the vacuum in the headspace tends to facilitate reconstitution. It is common to "back-fill" with nitrogen or one other inert fuel previous to totally seating the stoppers. The most important goal in developing a freeze-dried product is to assure the standard requirements are met not only initially but all through the shelf lifetime of the product. These high quality attributes embody full recovery of the exercise of the product after addition of water (called reconstitution), reconstitution time, suitably low levels of extraneous particulate matter, sterility, the absence of pyrogens, and residual water content material. In addition, nonetheless, process situations must be chosen to be able to maximize course of efficiency. Freeze-drying typically requires 2�3 days or longer from the beginning of freezing to the completion of secondary drying. Success in meeting the quality necessities of the product as well as minimizing inefficiencies within the course of requires a good understanding of formulation of freeze-dried merchandise, physical chemistry of freezing, the rules of heat and mass transfer, and process monitoring. Formulation of Freeze-Dried Products A good basic rule in developing a formulation of a freezedried pharmaceutical product, or any product for that matter, is to hold the formulation so easy as potential, and to not embrace any part and not using a clear rationale for doing so together with supporting knowledge. It is essential to have a transparent idea of the important high quality attributes of the product before beginning. Some attributes, corresponding to sterile, non-pyrogenic, and compliant with compendial necessities for seen and subvisible particulate matter are apparent. Complete recovery of the exercise current within the formulation previous to freeze-drying is always desired, however could not all the time be potential. Vaccines, for example, tend to lose some efficiency because of freeze-drying, however the critical factor right here is Freeze-Drying insoluble. This can occur both spontaneously in solution or on account of denaturation in response to adsorption to solid surfaces similar to tubing or filters, or adsorption to the air�water interface present during processing. The smaller the heel radius, the more the stress related to expansion of the frozen system throughout freezing is concentrated, which in turn promotes vial breakage. Amorphous excipients include disaccharides such as sucrose, trehalose, and infrequently, lactose or maltose. These excipients may play a double function in a formulation as both a bulking agent and, for proteins and other biologicals, as a stabilizer. In order to be effective, the stabilizer should be amorphous and stay so all through the shelf life of the product.