
Ditropan
| Contato
Página Inicial

"Cheap 2.5 mg ditropan with mastercard, gastritis high fiber diet".
Y. Thorek, M.S., Ph.D.
Co-Director, University of California, Riverside School of Medicine
The lymph node cortex is separated from the connective tissue capsule (3) by the subcapsular (marginal) sinus (4 gastritis diet ����� ditropan 5 mg buy on-line, 12) chronic gastritis yahoo answers cheap 2.5 mg ditropan. The cortex consists of B cell�rich 441 lymphatic nodules (13) situated adjoining to one another however separated by internodular connective tissue trabeculae (5 healthy liquid diet gastritis purchase ditropan 2.5 mg line, 14) and trabecular (cortical) sinuses (6) gastritis flare up diet purchase ditropan 5 mg on line. Some lymphatic nodules exhibit a central, light-staining germinal center (7, 15) surrounded by a denser-staining peripheral portion (13). In the germinal centers (7, 15), the cells are loosely aggregated with the developing lymphocytes showing larger and lighter-staining nuclei with more cytoplasm. The deeper portion of the lymph node cortex is the paracortex (8, 17), a thymus-dependent zone primarily occupied by T cells. This area represents the transition from the lymphatic nodules (7, 13) to the medullary cords (9, 19) of the lymph node medulla. The medulla consists of anastomosing cords of lymphatic tissue, the medullary cords (9, 19), interspersed with medullary sinuses (10, 18) that drain the lymph from the node into the efferent lymphatic vessels in the hilus. Fine reticular connective tissue offers support for the lymph node and types the core of the lymphatic nodules (13) in the cortex, the medullary cords (9, 19), and all medullary sinuses (10, 18) within the medulla. Most of the lymphocytes are small with giant, deep-staining nuclei with condensed chromatin and both a small quantity of cytoplasm or none in any respect. They have a strategic location alongside the paths of lymphatic vessels and are most outstanding in the inguinal and axillary regions. Their main capabilities are lymph filtration and the phagocytosis of micro organism or international substances from the filtered lymph, preventing them from reaching the overall circulation. Trapped within the reticular fiber network of every lymph node are fixed or free macrophages that destroy overseas substances. Thus, as lymph is filtered, the nodes localize and stop the unfold of infection into the general circulation and other organs. Here the lymphocytes proliferate, and the B cells can remodel into plasma cells. As a result, lymph that leaves the lymph nodes through the efferent vessels may comprise antibodies that might be distributed to the whole physique. In the lymph node, the B cells congregate within the lymphatic nodules in the outer cortex, 443 whereas the T cells concentrate beneath the lymphatic nodules in the deep cortical or paracortical (paracortex) regions. Lymph nodes are also the sites of antigenic recognition and antigenic activation of B cells, giving rise to plasma cells and reminiscence B cells. Lymphatic nodules that lack the light-staining germinal facilities and only exhibit the dense aggregations of lymphocytes are considered as inactive major lymphatic nodules. After antigenic stimulation, major lymphatic nodules turn out to be secondary lymphatic nodules with a lighter-staining germinal facilities surrounded by dense staining lymphocytes. Germinal facilities turn out to be the most important websites for varied B-cell proliferation and differentiation, whereas the T cells undergo the identical course of within the paracortex of the lymph node beneath and between the lymphatic nodules. Continuous lymphocyte circulation between blood and lymph takes place in the lymph nodes, tonsils, Peyer patches, and spleen. Lymph fashioned in the physique eventually reaches the blood, and lymphocytes that depart the lymph nodes via the efferent lymph vessels also return to the bloodstream. The arteries supplying lymph nodes branch into capillaries in the cortex and paracortex, which give an entryway for lymphocytes into the lymph nodes. Most lymphocytes enter the lymph nodes by way of the postcapillary venules within the paracortex. B cells and T cells acknowledge special adhesion molecules on the excessive endothelial cells in these venules and depart the bloodstream to enter the lymph node. This pathway allows the movement of lymphocytes to travel in lymph to other lymph nodes, ultimately coming into the systemic circulation. Movement of B cells and T cells across the excessive endothelial venules into lymph nodes is considered homing. These specialised venules are additionally present in Peyer patches within the small intestine, tonsils, appendix, and cortex of the thymus; excessive endothelial venules are absent from the spleen. A unfastened connective tissue capsule (4) with blood vessels and adipose cells (7) covers the lymph node. Inferior to the capsule (4) is the subcapsular (marginal) sinus (5), which overlies the darker-staining and peripheral lymph node cortex (3). The cortex (3) displays quite a few lymphatic nodules (1, 6), some with a lighter-staining germinal middle (2). The central area of the lymph node is the lighter-staining medulla (9), characterized by the dark-staining medullary cords (12) and the light-staining lymphatic channels, the medullary sinuses (11). The medullary sinuses (11) drain the lymph that enters the lymph node by way of the afferent lymphatic vessels in the capsule. The lymph leaves the lymph node via the efferent lymphatic vessels with valves (10) at the hilum. The reticular connective tissue, the reticular cells (8, 11), is seen in numerous 445 areas of the node. Reticular cells (8, 11) are visible within the subcapsular sinus (1), trabecular sinuses (12), and the germinal middle (9) of the lymphatic nodule (14). Numerous free macrophages (2, 6, 16) are also seen in the subcapsular sinus (1), trabecular sinuses (12), and the germinal heart (9) of the lymphatic nodule (14). A lymphatic nodule with a small part of its peripheral zone (14) and a germinal middle (9) with creating lymphocytes are seen. Endothelial cells (5, 13) line the sinuses (1, 12) and form an incomplete cowl over the surface of the lymphatic nodules (14). The dense peripheral zone of the lymphatic nodule (14) accommodates an aggregation of small lymphocytes (7), characterised by dark-staining nuclei, condensed chromatin, and little or no cytoplasm. Small lymphocytes (7) are also current in the subcapsular sinus (1) and trabecular sinuses (12). The germinal middle (9) of the lymphatic nodule (14) incorporates medium-sized lymphocytes (10) characterized by larger, lighter nuclei and more cytoplasm than in the small lymphocytes (7). The nuclei of medium-sized lymphocytes (10) exhibit variations in the size and density of the chromatin. The largest cells, with much less condensed chromatin, are the lymphoblasts (17) visible within the germinal heart (9) as massive cells with a broad band of cytoplasm and a large vesicular nucleus with one or more nucleoli. With mitotic divisions of lymphoblasts (15), the chromatin condenses and the cells decrease in size, producing small lymphocytes (7). This image exhibits a high endothelial venule (2) lined by tall cuboidal endothelium, instead of the standard squamous endothelium. Several migrating lymphocytes (3) are seen shifting via the venule wall between the high endothelium (2) into the paracortex. Surrounding the high endothelial venule (2) are lymphocytes of the paracortex (5), a medullary sinus (1), and a venule (4) with blood cells. The thicker and denser collagen fibers within the connective tissue capsule (3) stain pink. Both the capsule and the lymph node are supported by delicate reticular fibers (6, 9) that stain black and kind a fantastic meshwork throughout the organ. A connective tissue trabecula (4) from the capsule (3) penetrates the interior of the lymph node between two lymphatic nodules (8, 12). Inferior to the capsule (3) are subcapsular (marginal) sinuses (1, 7) that continue on both sides of the trabecula (4) as trabecular sinuses (2, 5) into the medulla of the node and eventually to exit by way of the efferent lymph vessels in the hilum. Each lobule consists of a dark-staining outer cortex (3, 13) and a light-staining inside medulla (4, 12). Because the lobules are incomplete, the medulla reveals continuity between the neighboring lobules (4, 12). Blood vessels (5, 14) cross into the thymus gland by way of the connective tissue capsule (1) and the trabeculae (2, 10). In distinction, the medulla (4, 12) contains fewer lymphocytes however more epithelial reticular cells. The medulla also incorporates numerous thymic (Hassall) corpuscles (6, 9) that characterize the thymus gland. By puberty, thymus glands start to involute with gradual regression and degeneration. As a consequence, lymphocyte production declines, and the thymic (Hassall) corpuscles (6, 9) become extra prominent. In addition, the parenchyma or mobile portion of the gland is 448 progressively changed by unfastened connective tissue (10) and adipose cells (7, 11). The thymus gland depicted on this illustration exhibits adipose tissue accumulation and signs of involution related to getting older. In contrast, the medulla (3) accommodates just a few lymphocytes but more epithelial reticular cells (7, 10).
Therefore gastritis diet journals ditropan 5 mg order otc, monitoring of creatinine clearance is required to optimize publicity and reduce toxicity gastritis diet ���� ditropan 5 mg discount with mastercard. Optimal treatment rates are noticed when trough concentrations are maintained between 10 and 20 mcg/mL gastritis length ditropan 2.5 mg low cost. Common antagonistic occasions embody nephrotoxicity gastritis diet ��������� cheap ditropan 2.5 mg without prescription, infusion-related reactions (red man syndrome and phlebitis), and ototoxicity. Resistance is pushed by alterations in binding affinity to peptidoglycan precursors. Lastly, vancomycin has poor absorption after oral administration, so use of the oral formulation is limited to the management of Clostridium difficile an infection within the colon. The lipoglycopeptides keep a spectrum of exercise just like vancomycin, affecting primarily staphylococci, streptococci, and enterococci. The lipid tail is important in anchoring the drug to the cell walls to improve goal site binding. In mixture, these actions enhance activity and minimize choice of resistance. Prior to initiation, assessment of renal function, pregnancy status, and current medications is needed to ensure safe administration. Oritavancin and telavancin are known to intrude with phospholipid reagents used in assessing coagulation. Efficacy of treatment with daptomycin in left-sided endocarditis has not been demonstrated. Additionally, daptomycin is inactivated by pulmonary surfactants; thus, it should never be used in the remedy of pneumonia. It blocks cell wall synthesis by inhibiting the enzyme enolpyruvyl transferase, a key step in peptidoglycan synthesis. Due to its distinctive structure and mechanism of motion, cross-resistance with other antimicrobial brokers is unlikely. The drug is excreted in its lively type within the urine and maintains excessive concentrations over several days, allowing for a one-time dose. Polymyxins the polymyxins are cation polypeptides that bind to phospholipids on the bacterial cell membrane of gram-negative micro organism. They have a detergent-like effect that disrupts cell membrane integrity, leading to leakage of cellular components and cell dying. However, alterations within the cell membrane, lipid polysaccharides permit many species of Proteus and Serratia to be intrinsically resistant. Polymyxin B is on the market in parenteral, ophthalmic, otic, and topical preparations. The use of those medication has been restricted due to the increased risk of nephrotoxicity and neurotoxicity (for instance, slurred speech, muscle weakness) when used systemically. Incision and drainage were performed on the abscess, and cultures revealed methicillin-resistant Staphylococcus aureus. Which is the most applicable therapy choice for once-daily outpatient intravenous therapy on this affected person Myalgias and rhabdomyolysis have been reported with daptomycin remedy and require patient education and monitoring. Which of the following regimens is most acceptable for empiric coverage of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa on this affected person Vancomycin + cefepime + ciprofloxacin Vancomycin + cefazolin + ciprofloxacin Telavancin + cefepime + ciprofloxacin Daptomycin + cefepime + ciprofloxacin Correct answer = A. The cephamycins (cefoxitin and cefotetan) are the only cephalosporins with in vitro activity in opposition to anaerobic gram-negative pathogens. Option C is the solely option since telavancin is approved for skin and pores and skin structure infections, and the patient has no apparent contraindication. Which of the following is an applicable oral option to treat the urinary tract infection on this affected person Option A and C are incorrect as a result of enterococci are inherently proof against all cephalosporins. Option D is the greatest choice, as amoxicillin is nicely absorbed orally and concentrates within the urine. Overview A number of antibiotics exert their antimicrobial results by targeting bacterial ribosomes and inhibiting bacterial protein synthesis. However, high concentrations of medication similar to chloramphenicol or the tetracyclines might cause toxic results on account of interaction with mitochondrial mammalian ribosomes, because the structure of mitochondrial ribosomes extra intently resembles bacterial ribosomes. Tetracyclines Tetracyclines consist of four fused rings with a system of conjugated double bonds. Mechanism of motion Tetracyclines enter prone organisms through passive diffusion and by an energy-dependent transport protein mechanism unique to the bacterial inside cytoplasmic membrane. Antibacterial spectrum the tetracyclines are bacteriostatic antibiotics effective in opposition to a broad variety of organisms, together with gram-positive and gram-negative micro organism, protozoa, spirochetes, mycobacteria, and atypical species. Other mechanisms of bacterial resistance to tetracyclines embrace enzymatic inactivation of the drug and manufacturing of bacterial proteins that forestall tetracyclines from binding to the ribosome. Distribution the tetracyclines focus nicely within the bile, liver, kidney, gingival fluid, and pores and skin. Moreover, they bind to tissues undergoing calcification (for example, enamel and bones) or to tumors which have high calcium content. Minocycline also achieves high concentrations in saliva and tears, rendering it helpful in eradicating the meningococcal carrier state. All tetracyclines cross the placental barrier and focus in fetal bones and dentition. Elimination Tetracycline is primarily eradicated unchanged within the urine, whereas minocycline undergoes hepatic metabolism and is eradicated to a lesser extent via the kidney. Esophagitis could additionally be minimized via coadministration with food (other than dairy products) or fluids and using capsules quite than tablets. Effects on calcified tissues Deposition within the bone and primary dentition happens through the calcification process in rising youngsters. Hepatotoxicity Rarely hepatotoxicity could happen with excessive doses, particularly in pregnant girls and those with preexisting hepatic dysfunction or renal impairment. Vestibular dysfunction Dizziness, vertigo, and tinnitus could happen significantly with minocycline, which concentrates within the endolymph of the ear and impacts perform. Pseudotumor cerebri Benign, intracranial hypertension characterised by headache and blurred imaginative and prescient may occur not often in adults. It is indicated for the treatment of sophisticated skin and delicate tissue infections, sophisticated intra-abdominal infections, and community-acquired pneumonia. Mechanism of motion Tigecycline reveals bacteriostatic action by reversibly binding to the 30S ribosomal subunit and inhibiting bacterial protein synthesis. Resistance Tigecycline was developed to overcome the emergence of tetracycline class�resistant organisms that utilize efflux pumps and ribosomal safety to confer resistance. Resistance to tigecycline has been observed and is primarily attributed to overexpression of efflux pumps. No dosage changes are needed for sufferers with renal impairment; nonetheless, a dose reduction is really helpful in severe hepatic dysfunction. All-cause mortality in sufferers treated with tigecycline is larger than with other agents. Aminoglycosides Aminoglycosides are used for the treatment of serious infections due to cardio gram-negative bacilli; however, their scientific utility is limited due to critical toxicities. Mechanism of action Aminoglycosides diffuse by way of porin channels in the outer membrane of vulnerable organisms. These organisms even have an oxygen-dependent system that transports the drug across the cytoplasmic membrane. Because of those properties, high-dose extended-interval dosing is usually utilized. This dosing strategy also reduces the risk of nephrotoxicity and increases comfort. Antibacterial spectrum the aminoglycosides are efficient for the majority of aerobic gram-negative bacilli, together with those which could be multidrug resistant, similar to Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterobacter sp. Resistance Resistance to aminoglycosides occurs by way of: 1) efflux pumps, 2) decreased uptake, and/or 3) modification and inactivation by plasmid-associated synthesis of enzymes. It is run topically for skin infections or orally to decontaminate the gastrointestinal tract prior to colorectal surgery. Distribution Because of their hydrophilicity, aminoglycoside tissue concentrations could additionally be subtherapeutic, and penetration into most physique fluids is variable.
The simple columnar floor epithelium (1 gastritis diet ditropan 5 mg cheap on line, 13) extends into the gastric pits (11) into which open the tubular gastric glands (5) gastritis icd 10 buy ditropan 2.5 mg line. The lamina propria (6) fills the spaces between the packed gastric glands (5) and extends from the floor epithelium (1) to the muscularis mucosae (9) gastritis dietz best 2.5 mg ditropan. The lamina propria (6) and collagen fibers are higher seen in the mucosal ridges (2) chronic gastritis zinc discount 2.5 mg ditropan fast delivery. Scattered all through the lamina propria (6) are the fibroblast nuclei, lymphoid lymphatic nodule (17), lymphocytes, and other loose connective tissue cells. The gastric glands (5) lengthen the length of the mucosa and in deeper areas the gastric glands may department. At the junction of the gastric pit with the gastric gland is the isthmus (14), lined with surface epithelial cells (1, 13) and parietal cells (4). Lower in the gland is the neck (15), containing primarily mucous neck cells (3) and some parietal cells (4). The base or fundus (16) is the deep portion of the gland, composed predominantly of chief (zymogenic) cells (7) and a few parietal cells (4). The fundic glands additionally include undifferentiated cells and enteroendocrine cells (not illustrated) that secrete different hormones to regulate the digestive system. The mucous neck cells (3) are located just below the gastric pits (11) and are interspersed between the parietal cells (4) in the neck area of the glands. The parietal cells (4) stain uniformly acidophilic (pink), which distinguishes them from different cells within the fundic glands. In distinction, the chief cells (zymogenic) (7) are basophilic and are distinguishable from the acidophilic parietal cells (4). The muscularis mucosae (9) in the stomach are composed of two thin strips of smooth muscle: the inside round layer (9a) and outer longitudinal layer (9b). In this illustration, the inside round layer (9a) is sectioned longitudinally, and the outer layer (9b) is sectioned transversely. Extending upward from the muscularis mucosae (9) to the surface epithelium (1, 13) are strands of easy muscle (8, 12). The stomach floor is lined with a mucus-secreting, easy columnar epithelium (1) that extends down into the gastric pits (2). The large, pale-staining cells within the gastric glands (5) are the acid-secreting parietal cells (3), that are extra quite a few within the higher regions of the gastric glands (5). The pylorus is essentially the most inferior, funnel-shaped region of the abdomen that terminates at the border of the small gut known as the duodenum. In the cardiac, the gastric pits are shallow, whereas within the pylorus, the gastric pits are deep. However, the histology of gastric glands in each areas is similar, and the cells are predominantly mucus secreting. In contrast, the gastric glands in the fundus and physique exhibit totally different histology and comprise three major cell varieties. The large polygonal cells with eosinophilic cytoplasm are the parietal cells which are primarily positioned in the upper half of the gastric glands, squeezed between gland cells. Located predominantly within the lower region of the gastric glands are basophilic staining cuboidal chief (zymogenic) cells. In addition to cells in gastric glands, the mucosa of the digestive tract also incorporates enteroendocrine or gastrointestinal endocrine cells which are part of the diffuse neuroendocrine system. These cells are distributed in several digestive organs and are located amongst and between exocrine cells. Unless digestive organs are ready with special stains, the diffuse neuroendocrine cells are tough to see in regular histologic sections. In addition, there are undifferentiated stem cells within the neck regions of the gastric glands that repeatedly renew the cells within the gastric mucosa. These stem cells move upward to replace lost or worn-out floor cells or downward to substitute the cells deep within the glands. The columnar floor epithelium (1) reveals basal oval nuclei and a flippantly stained cytoplasm owing to the presence of mucigen droplets. The epithelium (1) is separated from the lamina propria (3, 7, 8) by a thin basement membrane (2) that extends downward into the gastric pits (4). The gastric glands (5) lie in the lamina propria (3, 7, 8) under the gastric pits (4). The neck region of the gastric glands (5) is lined with mucous neck cells (10) which have spherical, basal nuclei. The 567 constricted necks of the gastric glands (5) open into the underside of the gastric pits (4). The parietal cells (6, 11) are large cells with a pyramidal shape, spherical nuclei, and acidophilic cytoplasm interspersed among the mucous neck cells (10). They are probably the most conspicuous cells in the gastric mucosa and are found predominantly in the upper third to upper half of the gastric glands (5). The free surfaces of parietal cells (6, 11) open into the lumen of the gastric glands (5). Deeper within the decrease half of the gastric glands (5) are the basophilic chief (zymogenic) cells (12), which additionally border on the lumen of the gland. In the higher areas of the gastric glands, the chief or zymogenic cells (6, 10) border the lumen of gastric glands (1, 9). A small lymphatic nodule (4) is located within the lamina propria (3) adjacent to the gastric glands (1, 9). The two layers of the muscularis mucosae (5), the inside round layer and the outer longitudinal layer, are seen under the gastric glands (1, 9). Strands of easy muscle (8) prolong upward from the muscularis mucosae (5) into the lamina propria (3, 7) between the gastric glands (1, 9). In addition, the abdomen cells secrete totally different hormones that regulate digestive capabilities. Some capabilities are designed particularly to scale back the mass of ingested food materials, or bolus, to a semiliquid mass 569 referred to as chyme. The reduction of bolus is performed by strong, muscular peristaltic contractions of the stomach wall when food enters the abdomen. With the pylorus closed, the muscular contractions churn and mix the abdomen contents with gastric juices produced by the gastric glands. Neurons and axons located within the submucosal nerve plexus and myenteric nerve plexus of the abdomen wall regulate the peristaltic activity. In addition, the abdomen additionally performs some absorption; nevertheless, that is limited to absorption of water, alcohol, salts, and sure medication. The mucus produced by these glands and the cardiac glands of the esophagus neutralize the gastric reflux and shield the esophageal lining. Chemical reduction or digestion of food in the stomach is the main perform of gastric secretions produced by different cells in the gastric glands, particularly by cells within the fundus and body of the abdomen. The main parts of the gastric secretions are pepsin, hydrochloric acid, mucus, gastric intrinsic issue, water, lysozyme, and different electrolytes. The floor or luminal epithelial cells of the stomach and the mucous neck cells of the gastric pits secrete thick layers of mucus. This secretion covers, lubricates, and protects the stomach floor from the corrosion by acidic gastric juices secreted by the gastric glands and the ingested material. Vitamin B12 is necessary for erythrocyte (red blood cell) production (erythropoiesis) in the purple bone marrow. Deficiency of this vitamin results in the development of pernicious anemia, a disorder of erythrocyte formation. Chief (zymogenic) cells are crammed with granules that contain the proenzyme pepsinogen, an inactive precursor of pepsin. Release of pepsinogen during gastric secretion into the acidic surroundings of the stomach converts the inactive pepsinogen into the highly active, proteolytic enzyme pepsin. This enzyme digests large protein molecules into smaller 570 peptides, changing almost all the proteins into smaller molecules. The secretory activities of the chief and parietal cells are controlled by the autonomic nervous system and the hormone gastrin, secreted by the enteroendocrine cells of the pyloric region of the stomach. Enteroendocrine cells secrete various polypeptides and proteins with hormonal exercise that influences digestive tract capabilities. They are called enteroendocrine cells as a end result of they produce gastric hormones and are situated within the digestive organs.
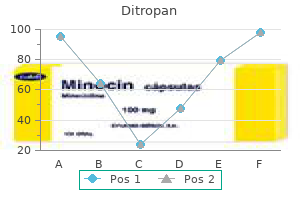
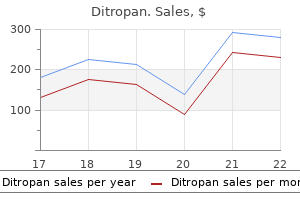
Ditropan 2.5 mg order online. Dr. ETV | Gastritis Diet | 29th March 2018 | డాక్టర్ ఈటివీ.
Syndromes
- Numbness or tingling
- Losing interest in things previously enjoyed, flat mood
- Vision impairment or blindness (with the early-onset forms of the disease)
- Stage 1 is called early localized Lyme disease. The infection has not yet spread throughout the body.
- Nummular eczema
- Narrowing of the cervical spine (spinal stenosis)
- Rapid breathing
- If this method fails, get medical help.
- Groin pain
- Acute kidney failure
These are the proximal convoluted tubules (1) and distal convoluted tubules (2 gastritis vs ulcer symptoms generic 5 mg ditropan, 4) atrophic gastritis symptoms mayo ditropan 2.5 mg cheap online. The proximal convoluted tubules (1) are longer than the distal convoluted tubules (2 gastritis y colitis 2.5 mg ditropan, 4) and are gastritis turmeric 5 mg ditropan purchase fast delivery, due to this fact, extra numerous in the cortex. The proximal convoluted tubules (1) exhibit a small, uneven lumen and a single layer of cuboidal cells with eosinophilic granular cytoplasm. The urinary capsular house (10) in the renal corpuscle (5, 9) is continuous with the lumen of the proximal convoluted tubule at the urinary pole. At the urinary pole, the squamous epithelium of the parietal layer (9b) of the glomerular capsule (5b) modifications to the cuboidal epithelium of the proximal convoluted tubule (1). The distal convoluted tubules (2, 4) are shorter and are fewer in number in the cortex. The distal convoluted tubules (2, 4) also exhibit bigger lumina with smaller cuboidal cells. Similar to the proximal convoluted tubules (1), the distal convoluted tubules (2, 4) show deep basal and lateral cell membrane infoldings and interdigitations. The medullary rays embody the following three kinds of tubules: straight (descending) segments of the proximal tubules (14), straight (ascending) segments of the distal tubules (6), and collecting tubules (12). The straight (descending) segments of the 703 proximal tubules (14) are similar to the proximal convoluted tubules (1), and the straight (ascending) segments of the distal tubules (6) are much like distal convoluted tubules (2, 4). The collecting tubules (12) in the cortex are distinct due to their frivolously stained cuboidal cells and distinct cell membranes. The medulla contains solely straight portions of the tubules and the segments of the loop of Henle (thick and skinny descending segments and thin and thick ascending segments). The skinny segments of the loops of Henle (15) are lined with a simple squamous epithelium and resemble the capillaries (13). The distinguishing options of the skinny loops of Henle (15) are the thicker epithelial lining and the absence of blood cells in their lumina. Also seen within the cortex are the interlobular blood vessels (3) and the larger interlobar vein and artery (7). The interlobular blood vessels (3) give rise to the afferent glomerular arteriole (11) that enters the glomerular capsule (5b) on the vascular pole (8) and types the capillary tuft of the glomerulus (5a). These cells are also hooked up to the capillaries and carry out several necessary functions. Mesangial cells synthesize the extracellular matrix and provide structural assist for the glomerular capillaries. As the blood is filtered via the glomerular 705 capillaries, numerous proteinaceous macromolecules are trapped in the glomerular basement membrane and filtration slit diaphragms. Mesangial cells perform as macrophages within the intraglomerular areas by removing the trapped material from filtration slits and glomerular basal membrane, thus preventing its clogging and maintaining the glomerular filter free of debris. They also phagocytose antigen�antibody complexes and produce several interleukins in response to glomerular damage or damage. Mesangial cells are also contractile and regulate glomerular blood move and strain changes within the vascular pole area, between the afferent and efferent arterioles. The kidneys also produce urine, which is formed by three primary capabilities: (1) filtration of blood within the glomeruli, (2) reabsorption of vitamins and different useful substances from the ultrafiltrate that enters the proximal and distal convoluted tubules, and (3) secretion, or excretion, of metabolic waste merchandise or undesirable chemicals or substances into the filtrate that turn out to be urine. Approximately 99% of the glomerular ultrafiltrate produced by the kidneys is reabsorbed into the system; the remaining 1% of the filtrate is voided as urine. In addition, kidneys have endocrine functions by producing hormones erythropoietin and renin. Endothelial cells of the peritubular capillary network within the renal cortex produce the glycoprotein hormone erythropoietin, which is a growth factor that stimulates erythrocyte production in purple bone marrow in response to hypoxia, a decrease in oxygen focus in blood or tissues. Renin is produced by kidney cells to regulate blood strain and to maintain correct filtration stress within the glomeruli. As the ultrafiltrate from 706 nephrons passes through the uriniferous and collecting tubules of the kidneys, it undergoes significant changes in its content and quantity producing concentrated urine, containing metabolic waste merchandise. The cells of the proximal convoluted tubules show deep infoldings of the basal cell membrane, between that are located elongated mitochondria and lateral membrane interdigitations with neighboring cells. These options are attribute of cells concerned in energetic transport of molecules and electrolytes from the filtrate across the cell membrane into the interstitium. As the glomerular filtrate enters the proximal convoluted tubules, all glucose, proteins, and amino acids; nearly all carbohydrates; and about 75% to 85% of water and sodium chloride ions are absorbed from the glomerular filtrate into the encircling interstitium and peritubular capillaries. The presence of long and intently spaced microvilli (brush border) on proximal convoluted tubule cells significantly will increase the floor space and facilitates absorption of the filtered material. In addition, the proximal convoluted tubules secrete sure metabolites, hydrogen, ammonia, dyes, and medicines such as penicillin from the body into the glomerular filtrate. The metabolic waste products urea and uric acid stay in the filtrate of the proximal convoluted tubules and are eliminated from the body within the urine. Loops of Henle the descending and ascending loops of Henle of the juxtaglomerular nephrons are long, lengthen deep into the medulla, and have completely different permeabilities and completely different features. As a outcome, hypertonic urine is produced in the tubules by an osmotic gradient in the surrounding interstitium from the cortex of the kidney to the information of the renal papillae. Sodium chloride and urea are transported and concentrated in the interstitial tissue of the kidney medulla via a fancy countercurrent multiplier system, which creates a high interstitial osmolarity deep in the medulla. The descending loop of Henle is permeable to water however a lot less to sodium chloride, whereas the skinny ascending limb is permeable to sodium 707 chloride however to not water. The hypertonicity (high osmotic pressure) of the extracellular fluid within the medulla interstitium removes water from the glomerular filtrate because it flows via the descending skinny tubules, thereby rising its sodium and chloride concentration. In the ascending skinny limb, water stays behind, whereas sodium chloride leaves the fluid and is concentrated within the interstitium. The countercurrent flow of ultrafiltrate in the descending and ascending thin loops of Henle produces a gradient of osmolarity in the interstitium of the medulla. The water that enters the interstitium is then eliminated by the countercurrent blood flow within the capillary loops of the vasa recta, thus sustaining the osmotic focus gradient in the medulla, leading to water conservation and urine focus. These capillary loops are permeable to water and take up the water from the medullary interstitium to return it to the systemic circulation. Distal Convoluted Tubules the distal convoluted tubules are shorter, less convoluted than the proximal tubules, and fewer regularly noticed within the cortex and close to the renal corpuscles. The basolateral membranes of distal convoluted tubule cells also show elevated cell membrane interdigitations and elongated mitochondria inside these infoldings. The distal convoluted tubules actively reabsorb sodium ions from the tubular filtrate and excrete hydrogen, potassium, and ammonium ions into the tubular fluid. The excretion of hydrogen ions is related with the absorption of bicarbonate ions and rising the acidification of urine. Sodium reabsorption within the distal convoluted tubules is managed by the hormone aldosterone secreted by the adrenal cortex. Aldosterone hormone induces cells of the distal convoluted tubules to actively take in sodium and chloride ions from the filtrate and transport them into the interstitium. From the interstitium, sodium chloride ions are shortly absorbed by the peritubular capillaries and returned again to the systemic circulation, thereby decreasing sodium loss in urine. These capabilities of the distal convoluted tubules are vital for maintaining the right acid�base stability of physique fluids and blood. In the middle is the renal corpuscle with glomerular capillaries (5), parietal (8a) and visceral (8b) layers (epithelium) of the glomerular (Bowman) capsule (8), and the capsular space (10) across the glomerulus. Surrounding the renal corpuscle are the proximal convoluted tubules (7) with brush borders and acidophilic cells. These tubules are distinguished from the distal convoluted tubules (1, 6) that exhibit smaller and fewer intensely stained cells that lack the comb borders. In contrast to the convoluted tubules, the cuboidal cells of the accumulating tubule (11) exhibit pale cytoplasm and distinct cell outlines. Each renal corpuscle exhibits a vascular pole the place the afferent glomerular arteriole (4) enters and the efferent glomerular arteriole exits the renal corpuscle. Inside the renal corpuscle, the glomerular arteriole varieties a network of glomerular capillaries (5). Here, the capsular area (10) becomes steady with the lumen of the proximal convoluted tubule (7). The plane of part via both the vascular and urinary poles is just sometimes seen in the kidney cortex. This illustration shows the glomerular arteriole (4) on one finish and the urinary pole (9) on the reverse end of the renal corpuscle. At the vascular pole, modified epithelioid cells with cytoplasmic granules substitute the graceful muscle cells in the tunica media of the afferent glomerular arteriole (4).