
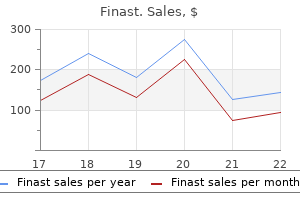
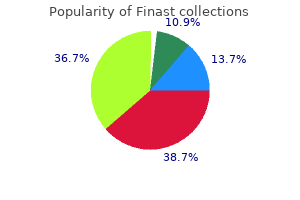
Finast
| Contato
Página Inicial

"Purchase finast 5 mg on line, hair loss cure garlic".
Z. Angir, M.A., M.D.
Clinical Director, University of Puerto Rico School of Medicine
The pH progressively increases to a maximum of approximately 7 at the ileocaecal junction hair loss herbal treatment finast 5 mg low price. In the colon the pH drops slightly because of the production of short-chain fatty acids by bacteria there hair loss from chemotherapy cheap 5 mg finast free shipping, but gradually rises once more distally hair loss cure yoga buy 5 mg finast amex. In some folks 567 What modified-release drug supply means for health care professionals and the pharmaceutical industry Provides physician gene therapy hair loss cure finast 5 mg generic without prescription, pharmacist and patient alternative. Stomach Small gut Colon Unit voided Transit time the time that a dosage type spends in the abdomen, small intestine and colon may be critical for some modified-release systems. Ingestion of meals delays this mechanism, and modified-release dosage forms can someday be trapped in the stomach so long as food is present. The small gut is the positioning of absorption for most medicine, and although the transit time of a dosage form through this region is normally around 3�4 hours, it might possibly really be extremely variable (from 0. A modified-release dosage kind which releases the drug very slowly must bear in mind that it may solely be at its site of absorption for a couple of hours. Often modified-release dosage types attain the colon (as they might not have disintegrated in the abdomen or small intestine). How efficient they will be at this level depends on whether or not the drug is absorbed in the colon. In one instance, it travelled slowly through the gut and the patient acquired an acceptable dose. Fluid Fluid levels could be highly variable in the abdomen, small intestine and colon. The colonic contents can be very viscous, with solely approximately 10 mL of free fluid actually available. All modified-release dosage varieties require the presence of fluid in order for drug release to occur. Less free liquid is out there because the modified-release dosage kind travels down the gut. The presence of ions, fat, enzymes and salts can all have an effect on the mechanisms of drug launch from modifiedrelease dosage types. For instance, fats could slow down launch from swelling matrix systems, meaning that the required blood ranges will not be achieved as quickly of their presence. Designing a modified-release formulation: components to think about There are a quantity of decisions that have to be taken when designing a modified-release formulation. Assuming it has been established that a drug is an appropriate candidate for modified-release drug supply, the factors mentioned in the following sections ought to be considered. A single-unit dosage type is advantageous from a manufacturing standpoint, as it could usually be manufactured using conventional strategies, such as compaction and film coating. For medicine targeted to the small or giant intestine, this might prevent them reaching their website of action. However, these can be more difficult to manufacture (requiring extrusion� spheronization or drug loading onto seed cores) and to scale up. Firstly, the release-modifying ingredients could be integrated all through the matrix of the dosage type, whereby the whole dosage kind encompasses the modified-release factor. The second option is the application of a modified-release coating to a dosage type, wherein the drug is normally contained within the core and is released by way of, or by way of the dissolution of, the modified-release coat. There are slight deviations from these two strategies nevertheless, as will be seen in later sections. These are (1) dissolution of the lively drug element and (2) diffusion of dissolved species. Hydration of the gadget (either swelling or dissolution of some component of the modified-release dosage form). There are various methods which have been adopted in an try and management and manipulate drug launch patterns. Extended launch Before an extended-release dosage form is developed, the suitability of the drug in question must be considered. The solubility of a drug in aqueous media, and its ability to permeate the gut, are key considerations when one is assessing whether a drug could additionally be suitable for modified release. There are three potential rate-limiting steps within the bioavailability of a drug from a dosage form: 1. The Biopharmaceutics Classification System of medicine (see Chapter 21 for details) classifies drugs into four categories: High-solubility and high-permeability medicine (class I) are most fitted for extended-release delivery (ideally by passive diffusion). These properties mean that drug launch from dosage types could be the ratelimiting step within the process, and this could then be tailor-made by the dosage type design. For medicine with low solubility (< 1 mg/mL), the low rate of dissolution can already give some inherent sustained-release behaviour of the pure drug molecule, and dissolution of drug particles in the gut may be the rate-limiting step. Drugs with long half-lives could obtain pseudo-sustained-release blood ranges regardless of being formulated as immediate-release types, whereas for drugs with shorter half-lives, very excessive doses may be needed to preserve blood levels. To restrict the size of the dosage kind, the efficiency of the drug in the modified-release form can be crucial. Up to a thousand mg potency tablets are available in extendedrelease formulations, but that is solely achieved by utilizing very large tablets, which can not all the time be acceptable for some affected person populations (especially paediatric or geriatric patients; see Chapter 45). Hydrophilic matrix systems Hydrophilic matrix systems may also be referred to as swellable soluble matrices. The drug is blended with a water-swellable, hydrophilic polymer (usually together with another excipient materials) and compressed right into a pill. The polymer is normally in the type of a powder or granule, and tablets shall be manufactured by direct compression or roller compaction (dry granulation processes). On publicity to fluid, the polymer materials in the pill starts to swell, producing a gel matrix. Drug in blood profiles (right graphs) are influenced by drug release, but also by absorption, distribution, metabolism and excretion and so drug levels in the blood rise and fall according to all these parameters combined. In the human body, these drug ranges take time to construct up within the blood to a stable level. This can then be followed by a sluggish release of the remaining drug (the upkeep dose) which ought to sustain the blood levels within the therapeutic vary. First-order launch kinetics can be utilized to obtain the primer dose in a fast style. Another instance of bimodal launch is delayed launch followed by extended launch. First order Slow/sustained launch of drug is achieved by diffusion via a nondissolving sponge-like polymer scaffold Insoluble polymers. Tableting Yes No Modified-release oral drug delivery Pharmaceutical know-how Commercial products Tableting Status Commercial products Use Drugs requiring extended-release action to obtain once-daily dosing, reduced toxicity, etc. The price at which water can diffuse by way of the pill � and later by way of the hydrated gel � impacts the drug release fee. Hydrophilic gels can be considered a community of interlinked/interspersed polymer stands. In the interstitial spaces between the strands is a steady section through which water and drug might diffuse. This can be affected by utilizing polymers of different molecular weights or through the use of cross-linked gels, and so the release rate may be modified by these components. Diffusionbased launch mechanisms normally observe zero-order or first-order kinetics (assuming sink conditions within the gastrointestinal tract and enough fluid), but further erosion of the matrix due to gastrointestinal motility and hydrodynamics can complicate the true in vivo release price. Hydrophilic matrix systems would generally be chosen where a sustained drug launch is required. This usually results from the problem of the gastrointestinal surroundings, which is variable with respect to fluid, food and transit. Membrane-controlled systems Membrane�controlled supply methods differ from the matrix formulations in that the rate-controlling part of the system is a membrane through which the drug should diffuse, somewhat than diffusing by way of the whole matrix. Generally, drug is concentrated in the core, and must traverse a polymeric membrane or movie, which slows down the release fee. Drug launch via a membrane is controlled by the thickness and the porosity of the membrane, in addition to the solubility of the drug within the gastrointestinal fluids. The biopharmaceutical considerations of transit and fluid are a lot the identical as for monolithic matrix tablets. However, membrane-controlled drug delivery methods could additionally be extra prone to be within the type of pellets than monolithic techniques. For example, tablets are more probably to turn into trapped in the stomach if administered with food (especially with a high-calorie meal). They also have less threat of dose dumping; if a tablet coating fails, then the entire dose can be dumped; with a pellet formulation, the disruption Insoluble polymer matrix these are far much less commonly used than their watersoluble/water-swellable counterparts. They encompass an inert matrix system by which the drug is embedded in an inert polymer.
This method is equal to two one-sided checks with the null speculation of bioinequivalence at the 5% significance stage hair loss in men volleyball purchase finast 5 mg visa. These formulations differ by way of their rates of absorption (tmax and Cmax are different); nevertheless hair loss cure wikipedia finast 5 mg discount overnight delivery, for each formulations the drug concentration is below the utmost secure concentration hair loss cure 2016 finast 5 mg buy with amex, so no huge difference in tolerability could be expected and the 2 formulations are above the minimum effective focus for the same interval so can be expected to perform similarly hair loss kittens cheap 5 mg finast mastercard. In the case of drug merchandise containing a drug which displays a narrow vary between its minimum effective plasma focus and its maximum safe plasma focus. Bioequivalence research for products corresponding to this, with slender therapeutic windows, may well require tighter statistical limits. This can normally be carried out to help minor changes to the formulation, process or scale during improvement, to examine different strengths of the formulation that have the same qualitative and quantitative composition and to justify minor changes in the formulation and manufacture after approval. The Biopharmaceutics Classification Scheme (see later) can be utilized to help justify the utilization of dissolution testing to decide bioequivalence. Normally, a representative number of dosage forms have to be tested (at least 12), and dissolution profiles of the product must be generated on all strengths using an acceptable dissolution methodology in three media. The pharmacodynamic parameters measured must be relevant to the therapeutic impact and correlate with the efficacy of the drug and, probably, safety. A pharmacodynamic effect dose�response curve is required so that it can be ensured that variations in formulation shall be distinguished and no maximal impact of the response is more probably to be seen through the examine. The response needs to be measured quantitatively under double-blind situations, repeatedly, so that the pharmacodynamic occasion may be precisely recorded. As for pharmacokinetic research the assay method must be precise, accurate, reproducible and particular. Where no pharmacokinetic or pharmacodynamics parameters can be measured, as within the case of merchandise intended for native motion, not involving systemic absorption. Again, careful research design is required to guarantee the proper number of individuals, medical finish points and potential security finish factors are achieved. There is likely to be higher variability with scientific research than with pharmacokinetic studies. The indicators are assembled by computer software program to kind a two-dimensional picture of the dosage form in the gastrointestinal tract. The anatomy of the gastrointestinal tract can be clearly seen from liquid dosage types, and the positioning of disintegration of strong dosage forms may be identified. One can measure the discharge of the radiolabel from the dosage form by following the depth of the radiation. By coadministration of a radiolabelled marker and a drug in the same dosage type, and simultaneous imaging and the taking of blood samples, the absorption site and launch fee of a drug can be decided. When used in this way, the technique is often referred to as pharmacoscintigraphy. Biopharmaceutics classification system As a results of the plethora and variability of biopharmaceutical properties of present and potential medicine, an attempt has been made to classify medicine into a small variety of classes. The scheme was initially proposed for the identification of immediate-release stable oral products for which in vivo bioequivalence tests will not be necessary. It can additionally be useful to classify medicine and predict bioavailability points that may arise through the varied phases of the development course of and is now used broadly by many regulatory authorities. The four lessons are outlined in phrases of excessive and low aqueous solubility and excessive and low permeability: Assessment of site of launch in vivo There are many benefits of being ready to assess the fate of a dosage form in vivo, and the positioning and release pattern of the drug. Gamma scintigraphy is now used extensively and permits higher data and understanding of the transit and fate of pharmaceuticals in the gastrointestinal tract to be gained. The method entails the radiolabelling of a dosage form with a -emitting isotope of appropriate half-life and exercise. Technetium-99m is commonly the isotope of selection for pharmaceutical studies due to its short half-life (6 h). A drug is taken into account to be extremely soluble when the highest dose strength is soluble in 250 mL or less of an aqueous medium over the pH vary from 1 to 8. The quantity is derived from the minimal volume anticipated in the abdomen when a dosage type is taken within the fasted state with a glass of water. The classification subsequently takes under consideration the dose of the drug as well as its solubility. A drug is taken into account to be highly permeable when the extent of absorption in people is expected to be greater than 90% of the administered dose. Permeability can be assessed with one of many methods mentioned earlier on this chapter that has been calibrated with identified normal compounds, or by pharmacokinetic studies. Although the 2 classification methods can be utilized to complement each other and each have the goal of speeding, simplifying and enhancing drug development, the purpose of the two classification techniques may be very totally different. Class I medicine will dissolve rapidly when offered in immediate-release dosage forms, and are additionally rapidly transported throughout the intestine wall. This class of drug must be amenable to formulation approaches to enhance the dissolution rate and therefore oral bioavailability. The concepts of bioequivalence and the Biopharmaceutics Classification System of medicine had been launched. It is imperative that the biopharmaceutical properties of medicine are fully understood, each in the choice of candidate medication in the course of the discovery process and in the design and growth of efficacious immediate-release and controlled-release dosage forms. One-compartment pharmacokinetic modelling can be used in scientific pharmacokinetic interpretation of drug ranges, offering blood sampling is finished after distribution. Most medicine show linear pharmacokinetic processes, where the rate of elimination is proportional to the plasma concentration. Some medication such as phenytoin, high-dose theophylline or salicylates and alcohol present nonlinear drug handling, where increased or multiple doses of a drugs may cause deviations from a linear pharmacokinetic profile and toxicity. The plasma�concentration time profile for a dosage form is influenced by the route of administration and the sort of formulation. At the regular state, the plasma focus fluctuates between a most and a minimal degree within a dosing interval. Changing the dose and the dosing interval will influence the extent of the fluctuations, in addition to the whole focus of the drug in the body. Dosage regimens: affect on the plasma concentration-time profile of a drug in the body the design of a dosage routine determines the therapeutic benefit for sufferers. The ideas of medical pharmacokinetics are utilized to design a dosage regimen for a patient that ensures the suitable formulation of the drug is chosen for an acceptable route of administration. The pharmacist needs to guarantee the suitable routine is prescribed to obtain optimum efficacy and minimal toxicity. Clinical pharmacokinetics provides a basic understanding of the ideas required to design a dosage regimen. Pharmacokinetics supplies a mathematical basis to assess the time course of drugs and their concentrations within the body. It allows the next processes to be quantified: � absorption; � distribution; � metabolism; and � excretion. The affect that physiological components, physicochemical properties of a drug, and dosage kind elements can have in figuring out whether or not a therapeutically efficient focus of a drug is achieved in the plasma following oral administration of a single dose of the drug is discussed in Chapters 19 and 20. For example, for 364 the treatment of a respiratory tract infection, amoxicillin could additionally be prescribed as one 500 mg capsule three times a day. Moreover, the dosage form have to be acceptable; as an example, a liquid could additionally be preferable to a tough capsule for young and older sufferers or sufferers with swallowing difficulties. In zero-order reactions the reaction proceeds at a relentless rate and is unbiased of the concentration of a substance current in the physique. Drugs exhibiting this sort of elimination will show accumulation of plasma ranges of the drug, and hence nonlinear pharmacokinetics. In first-order reactions the response proceeds at a price which relies on the focus of a drug in the physique. The majority of medicine used clinically at therapeutic dosages will exhibit first-order price processes, i. Whether a drug exhibits first-order or zero-order elimination is set by its Michaelis fixed (Km). This parameter is the plasma concentration at which the elimination of the drug proceeds at half the maximum metabolic capability (Vm). For most drugs, the Michaelis constant is far greater than the levels achieved by way of regular therapeutic use. Pharmacokinetic fashions are hypothetical constructs which describe the fate of a drug in a biological system following its administration. These fundamental parameters describe the fate of the drug following administration and are used to optimize a dosage routine. In a one-compartment mannequin the drug is considered to be distributed instantly all through the entire physique following its launch and absorption from the dosage type. Thus the physique behaves as a single compartment by which absorbed drug is distributed so rapidly that a concentration equilibrium exists at any given time between the plasma, other physique fluids and the tissues into which the drug has turn out to be distributed.
Finast 5 mg order without a prescription. What Is Baldness in Women? | Thinning Hair.
Pharmacopoeial checks the pharmacopoeial checks for assessing the quality of most powdered and granular dosage forms are very related hair loss cure latisse 5 mg finast buy with amex. The role of these checks is indicated by its title; details of procedures and standards may be discovered in the latest appropriate pharmacopeia hair loss in toddlers purchase finast 5 mg online. Single-dose oral powders should comply with a pharmacopoeial test for uniformity of dosage units or hair loss in men red 5 mg finast generic, where justified and approved hair loss cure jak inhibitor discount finast 5 mg, with the checks for uniformity of content and/or uniformity of mass. Pharmaceutical know-how of granule manufacturing Pharmaceutical granulation processes Granulation strategies can be divided into two varieties: moist methods, which use a liquid within the process, and dry methods, during which no liquid is used. Single-dose oral powders have to comply with a take a look at for uniformity of mass of single-dose preparations. The frequent types used are diluents, which are used to produce a unit dose weight of appropriate dimension, and disintegrating agents, that are added to help the break-up of the granule when it reaches a liquid medium. An adhesive (also known as a binder) within the form of a dry powder may be added, notably if dry granulation is employed. Dry granulation In the dry methods of granulation, the first powder particles are aggregated at excessive strain. In each cases the intermediate product is damaged using an acceptable milling approach to produce granular material which is often sieved to separate the specified dimension fraction. Organic solvents are used as an different to dry granulation when water-sensitive medicine are processed, or when a speedy drying time is required. In the normal wet granulation technique, the wet mass is compelled via a sieve to produce moist granules, that are then dried. A subsequent screening stage breaks agglomerates of granules and removes the nice material, which could be recycled. Variations of this traditional method are dependent on the gear used, but the general precept of preliminary particle aggregation utilizing a liquid stays in the entire processes. An different to the standard wet granulation course of is melt granulation whereby thermosetting polymers are used to kind the granules. Effect of the granulation technique on granule structure the type and capacity of granulating mixers considerably affect the work input and time essential to produce a cohesive mass, adequate liquid distribution and intragranular porosity of the granular mass. The technique and conditions of granulation affect intragranular pore construction by altering the diploma of packing within particular person granules. It has been shown that precompacted granules, consisting of drug and binder particles, are held together by simple bonding formed throughout consolidation. Granules ready by moist massing encompass intact drug particles held together in a sponge-like matrix of binder. Fluidized-bed granules are just like granules prepared by the wet-massing course of but possess higher porosity, and the granule floor is roofed by a film of binding agent. With spray-dried systems, the granules consist of spherical particles composed of an outer shell with an inside core of particles or air. Wet granulation (involving moist massing) Wet granulation involves the massing of a combination of dry main powder particles using a granulating fluid. The granulating fluid accommodates a solvent that must be volatile, in order that it could be removed by drying, and nontoxic. Typical appropriate liquids embody water, ethanol and 2-propanol both alone or in combination. The granulation liquid could additionally be used alone or, more normally, as a solvent containing a dissolved adhesive (also referred to as a binder or binding agent), which is used to guarantee particle adhesion once the granule is dry. The disadvantages of water as a solvent are that it might adversely have an effect on drug stability, inflicting hydrolysis of vulnerable merchandise, and it wants an extended drying time than organic solvents. This lengthy drying time increases the duration of the method and once more may have an effect on chemical stability of the drug(s) due to the prolonged publicity to warmth. The ones mentioned subsequent are these that are most relevant to pharmaceutical granulations. Adhesion and cohesion forces in motionless films If sufficient liquid is current in a powder to type a very thin, immobile layer, there shall be an effective lower in interparticulate distance and a rise involved area between the particles. The bond strength between the particles will be elevated because of this, because the van der Waals forces of attraction are proportional to the particle diameter and inversely proportional to the square of the gap of separation. This situation will come up with adsorbed moisture and accounts for the cohesion of slightly damp powders. In dry granulation, nevertheless, the pressures used will improve the contact area between the adsorbed layers and reduce the interparticulate distance, and it will contribute to the ultimate granule strength. Thin, immobile layers may be formed by extremely viscous options of adhesives. The resulting bond energy will be greater than that produced by the mobile movies discussed next. The use of starch mucilage in pharmaceutical granulations may produce this type of film. Sufficient liquid is usually added to exceed that needed for an immobile layer and this produces a mobile film. At low moisture ranges, termed the pendular state, the particles are held together by lens-shaped rings of liquid. These cause adhesion because of the surface rigidity forces of the liquid�air interface and the hydrostatic suction pressure in the liquid bridge. When all the air has been displaced from between the particles, the capillary state is reached, and the particles are held by capillary suction at the liquid�air interface, which is now only at the granule surface. The funicular state represents an intermediate stage between the pendular and capillary states. Moist granule tensile power increases by approximately 3 times from the pendular state to the capillary state. It could appear that the state of the powder bed depends upon the total moisture content material of the wetted powders however the capillary state may be reached by decreasing the separation of the particles. In the massing course of during moist granulation, continued kneading/mixing of fabric originally within the pendular state will densify the wet mass, lowering the pore quantity occupied by air and ultimately producing the funicular or capillary state with out further liquid addition. In this state the energy of the droplet relies upon the floor pressure of the liquid used. These moist bridges are only temporary constructions in wet granulation as a result of the moist granules will be dried. They are, nonetheless, a prerequisite for the formation of solid bridges fashioned by adhesives present in the liquid, or by materials which dissolve in the granulating liquid. Attractive forces between solid particles In the absence of liquids and strong bridges shaped by binding brokers, there are two kinds of attractive pressure which can operate between particles in pharmaceutical techniques. Electrostatic forces could also be of importance in inflicting powder cohesion and the initial formation of agglomerates. Van der Waals forces, nonetheless, are approximately 4 orders of magnitude stronger than electrostatic forces and contribute considerably to the strength of granules produced by dry granulation. The magnitude of those forces will enhance as the gap between adjoining surfaces decreases, and in dry granulation that is achieved with use of pressure to drive the particles together. Solid bridges these could be formed by: � partial melting; � hardening binders; or � crystallization of dissolved substances. When the stress is relieved, crystallization will happen, binding the particles collectively. Mechanisms of granule formation In the dry strategies, adhesion of particles happens because of applied pressure. A compact or sheet is produced which is bigger than the granule measurement required and due to this fact the required dimension can be attained by milling and sieving. In wet granulation methods, liquid added to dry powders has to be distributed throughout the powder by the mechanical agitation produced in the granulator. The particles adhere to one another due to liquid films, and additional agitation and/or liquid addition causes more particles to adhere. The precise mechanism by which a dry powder is remodeled into a bed of granules varies for every type of granulation equipment but the mechanism discussed in the following sections serves as a helpful broad generalization of the method. The proposed granulation mechanism may be divided into three phases: nucleation, transition and ball progress. This is the common mechanism in pharmaceutical moist granulations when an adhesive is included in the granulating solvent. The liquid will kind liquid bridges, as discussed earlier, and the adhesive will harden or crystallize on drying to form strong bridges to bind the particles. Adhesives similar to polyvinylpyrrolidone, the cellulose derivatives (such as carboxymethylcellulose) and pregelatinized starch perform on this method. When the granules are dried, crystallization of this materials will occur and the dissolved substance then acts as a hardening binder.
Three mechanisms are primarily responsible for particulate deposition within the lung: gravitational sedimentation hair loss cure stem cell 2013 5 mg finast cheap otc, impaction and diffusion hair loss cure4kids discount finast 5 mg free shipping. Inertial impaction the airstream modifications direction in the throat hair loss cure 2 finast 5 mg discount line, or where a bifurcation happens in the respiratory tract hair loss in men 55 finast 5 mg order otc. This deposition mechanism is particularly necessary for big particles having a diameter greater than 5 �m, and notably larger than 10 �m, and is common in the upper airways, being the principal mechanism for deposition within the nose, mouth, pharynx, larynx and the massive conducting airways. With the continuous branching of the conducting airways, the speed of the airstream decreases and impaction becomes a less necessary mechanism for deposition. Those larger than 5 �m will deposit predominantly by inertial impaction within the higher airways. Particles of dimension between 1 �m and 5 �m deposit primarily by gravitational sedimentation within the decrease airways, especially throughout slow, deep respiration, and particles smaller than 1 �m deposit by Brownian diffusion within the stagnant air of the decrease airways. This measurement of minimal deposition should thus be thought-about throughout formulation, though for the explanations of environmental humidity mentioned beforehand, the equilibrium diameter in the airways may be significantly bigger than the unique particle dimension within the formulation. Thus gravitational sedimentation of an inhaled particle depends on its dimension and density, in addition to its residence time within the airways. Sedimentation is an important deposition mechanism for particles in the size range from 0. Brownian diffusion Collision and bombardment of small particles by molecules in the respiratory tract produce Brownian motion. Breathing patterns Patient-dependent elements, such as breathing patterns, lung physiology and the presence of pulmonary illness, additionally affect particle deposition. For occasion, the larger the inhaled volume, the larger the peripheral distribution of particles in the lung, while rising the inhalation flow rate enhances deposition within the bigger airways by inertial impaction. Breathholding after inhalation increases the deposition of particles by sedimentation and diffusion. Optimal aerosol deposition happens with gradual, deep inhalations to whole lung capability, adopted by breath-holding prior to exhalation. Other mechanisms of deposition Although impaction, sedimentation and diffusion are the most important mechanisms for drug deposition in the respiratory tract, other mechanisms might occur. The composition of mucus and the process of mucociliary clearance are discussed in Chapter 38. Alveolar macrophages engulf such particles and may then migrate to the bottom of the mucociliary escalator, or could also be eliminated by way of the lymphatics. Hydrophobic compounds are normally absorbed at a rate dependent on their oil�water partition coefficients, whereas hydrophilic materials are poorly absorbed by way of membrane pores at rates inversely proportional to molecular dimension. Some medicine, such as sodium cromoglicate, are partly absorbed by a saturable energetic transport mechanism, whilst massive macromolecules could additionally be absorbed by transcytosis. The rate of drug absorption, and consequently drug motion, can be influenced by the formulation. Rapid drug action can usually be achieved using options or powders of aqueous soluble salts, whereas slower or extended absorption could also be achieved with suspension formulations, powders of much less soluble salts or novel drug delivery systems similar to liposomes, microspheres and nanocarriers. The head house of the aerosol canister is crammed with propellant vapour, producing the saturation vapour strain of the propellant at that temperature. On spraying, medicament and propellant are expelled, and the top quantity will increase. To reestablish the equilibrium, more propellant evaporates, and so a constant-pressure system with constant spray traits is produced. When released from the canister, the formulation undergoes quantity enlargement within the passage inside the valve and types a mixture of gasoline and liquid earlier than discharge from the orifice. The high-speed gasoline circulate helps to break up the liquid right into a fantastic spray of droplets. Containers Pharmaceutical aerosols could additionally be packaged in tin-plated metal, plastic-coated glass or aluminium containers. Alternatively, nontoxic compressed gases such as nitrogen dioxide, nitrogen and carbon dioxide may be used. However, ethanol has low volatility, and its inclusion could, depending on its concentration, enhance the droplet measurement of the emitted aerosols. Depression of the valve stem permits the contents of the metering chamber to be discharged via the orifice in the valve stem and made available to the affected person. After actuation, the metering chamber refills with liquid from the bulk and is in a position to dispense the following dose. The valve stem suits into the actuator, which is made from polyethylene or polypropylene. The dimensions of the orifice within the actuator play an important role, along with the propellant vapour strain, in determining the form and speed of the emitted aerosol plume. In chilly filling, drug substance, excipients and propellant are chilled, and the canister is filled with them at approximately -60 �C. Additional propellant is then added at the identical temperature, and the canister is sealed with the valve. In strain filling, most regularly employed for inhalation aerosols, a concentrated resolution or suspension of drug in propellant, underneath strain, is filled into canisters via the valve, adopted by addition of additional propellant. Once crammed, the canisters are leak examined, usually by inserting them in a water bathtub at elevated temperature, usually 50 �C to 60 �C. Following storage to enable equilibration of the formulation and valve elements, the containers are weighed to examine them for additional leakage, prior to insertion into actuators and spray testing. A cosolvent similar to ethanol or 2-propanol could additionally be used, although their low volatility retards propellant evaporation. In follow, the massive majority of pressurized inhaler formulations have been suspensions. These three-phase systems are tougher to formulate, and all the problems of conventional suspension formulation, similar to caking, agglomeration and particle growth, have to be thought-about. Careful consideration must also be given to the particle dimension of the solid (usually micronized to between 2 �m and 5 �m), valve clogging, moisture content material, the solubility of the drug substance within the propellant (a salt could additionally be desirable), the relative densities of the propellant and the drug, and the use of surfactants as suspending brokers. Solution formulations of some medication, corresponding to beclometasone dipropionate, are now available. The dose may be adjusted accordingly or the volatility of the product may be modified by the addition of a less volatile element, corresponding to glycerol. Many doses (up to 200) are stored within the small canister, which may also have a dose counter, and dose supply is reproducible. The inert circumstances created by the propellant vapour, together with the hermetically sealed container, defend medicine from oxidative degradation and microbiological contamination. On actuation, the primary propellant droplets exit at a high velocity, which may exceed 30 m s-1. Consequently, much of the drug is lost by way of impaction of these droplets in the oropharyngeal areas. The imply emitted droplet measurement typically exceeds 40 �m, and propellants may not evaporate sufficiently quickly for their size to lower to that suitable for deep lung deposition. Evaporation, such that the aerodynamic diameter of the particles is close to that of the unique micronized drug, may not occur till 5 seconds after actuation. Correct use by patients is vital for efficient drug deposition and therapeutic motion. However, it must be noted that even using the right inhalation method, solely 10% to 20% of the said emitted dose could also be delivered to the positioning of action. Volumatic, GlaxoSmithKline), although smaller, medium-volume spacers at the moment are obtainable. In the Autohaler (3M), an inspiratory demand valve triggers a spring mechanism to launch the drug, while in the Easi-Breathe (Teva), a vacuum within the gadget is launched on inspiration to trigger the actuation. The drug is either preloaded in an inhalation device or stuffed into hard gelatin or hypromellose capsules which are loaded into a tool previous to use. An enhance in turbulent airflow created by an increase in inhaled air velocity will increase the deaggregation of the rising particles but also increases the potential for inertial impaction in the upper airways and throat, and so a compromise has to be discovered. This reduces the preliminary droplet velocity, giant droplets could also be eliminated by impaction, efficient propellant evaporation occurs and the necessity for actuation�inhalation coordination is removed. The high-energy powders produced by micronization have poor move properties because of their static, cohesive and adhesive nature. The flowability of a powder is affected by its bodily properties, together with particle dimension and form, density, surface roughness, hardness, moisture content material and bulk density.